In the spectral series of hydrogen, as the wavelength decreases the lines appear closer together. What could be the reason for this? - Quora
4.7 (447) In stock
What should be the minimum energy of an electron exciting the
What is one of the causes of the breadth of spectral lines? - Quora
The number of spectral lines formed by a transition having an
How does a molecule produce a specific spectral pattern? - Quora
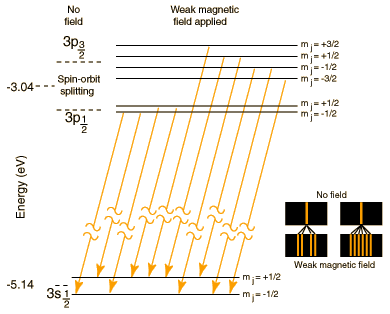
What is the cause of fine splitting of spectral lines? - Quora
Why do the spectral lines in the hydrogen atom become closer
Which spectral series of hydrogen spectrum lies in the visible
Spectral line - Wikipedia
In the spectral series of hydrogen, as the wavelength decreases the lines appear closer together. What could be the reason for this? - Quora
Why is the emission spectrum for hydrogen described by the Balmer
Why do spectral lines within a series decrease as the wavelength
If a hydrogen atom has only one electron, then why does it show
6.4: Emission and Absorbance Spectra - Chemistry LibreTexts
Calculating the Emission Spectra from Common Light Sources
 Удилище Daiwa Ballistic X Spin BLXS 40G (2.7/10-40)
Удилище Daiwa Ballistic X Spin BLXS 40G (2.7/10-40) Top 5 Fly Screen FAQ's Down Under Insect Screens & Security
Top 5 Fly Screen FAQ's Down Under Insect Screens & Security IP66 Waterproof Junction Box Plastic Large Electrical Enclosure Project Gray DIY
IP66 Waterproof Junction Box Plastic Large Electrical Enclosure Project Gray DIY RSR Casting Slab Spoon Baits — Lake Pro Tackle
RSR Casting Slab Spoon Baits — Lake Pro Tackle- AB Marine - SHAFT SHARK - Protect your drive train and propeller
 Refurbished) boAt Storm Pro with 1.78 AMOLED Display, 700+ Active
Refurbished) boAt Storm Pro with 1.78 AMOLED Display, 700+ Active
