CLIA Hot Topics CLIA Gary Yamamoto - ppt download
4.6 (271) In stock

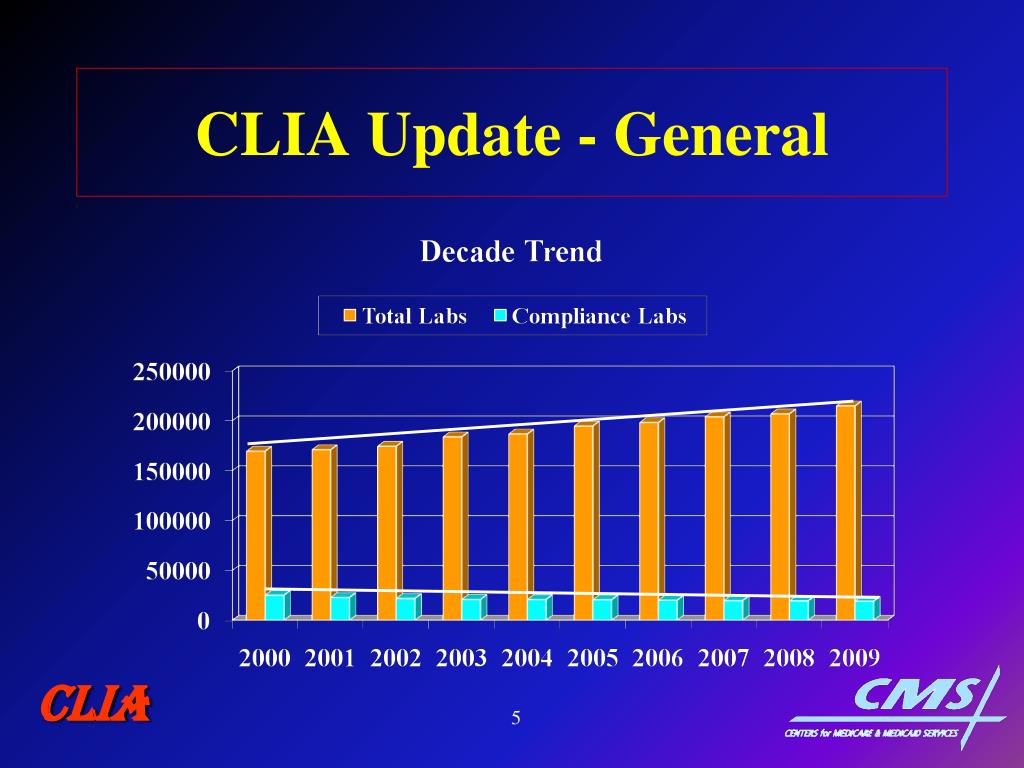
STATISTICS CLIA Certification Certificates Region IX Nation Waived 22,171 73% 181,270 71% PPMP 4,311 14% 36,255 Compliance 2,073 7% 19,553 8% Accreditation 1,712 6% 16,683 TOTAL 30, ,761 CLIA
CLIA Hot Topics CLIA Gary Yamamoto
Centers for Medicare & Medicaid Services. San Francisco Regional Office. CLIA.
Certificates. Region IX. Nation. Waived. 22, % 181, % PPMP. 4, % 36,255. Compliance. 2,073. 7% 19,553. 8% Accreditation. 1,712. 6% 16,683. TOTAL. 30, ,761. CLIA.
Region IX. Nation. POL. 50% 48% Pharmacy. 4% Nursing Facility. 6% Home Health Agency. Hospital. 3% Community Clinic. Independent. 2% CLIA.
Certificates. Nation. Waived. 72, % PPMP. 29, % Compliance. 12, % Accreditation. 5,856. 5% TOTAL. 119,293. CLIA.
Organization. Nation. COLA. 44% CAP. 39% TJC. 15% AABB. 1% ASHI. 0.4% AOA. 0.6% CLIA.
Replaces Equivalent Quality Control (EQC) CLIA.
Unless CMS approves a procedure specified in Appendix C of the State Operations Manual (CMS Pub. 7), that provides equivalent quality testing, the laboratory must. . . CLIA.
On January 1, 2016, laboratories must be in compliance with their quality control choice or deficiencies will be cited. CLIA.
May or may not reduce QC amount or frequency. IQCP is optional; default is regulations. CLIA.
CLIA certified laboratories should: Continue to follow existing quality control protocols. Decide to implement IQCP or default quality control. Plan and complete their transition accordingly, phasing out EQC, if applicable. CLIA.
Brochure 11: CLIA Individualized Quality Control Plan Introduction. Brochure 12: Considerations When Deciding to Develop an IQCP. Brochure 13: What is IQCP CLIA.
CMS, in collaboration with the CDC, released the IQCP Workbook. Geared primarily towards physician office laboratories (POL) and other smaller laboratories. CLIA.
Revised guidelines published on the CLIA website January 9, Summary of major changes included in S&C Memo CLIA. January 2016 revision: Removal of EQC. Addition of IQCP. CLIA.
For the following requirements, references to CLSI documents have been removed: 42 C.F.R. § (e)(4) [Media QC] 42 C.F.R. § (b)(1 – 2) [Susceptibility] For additional information, refer to S&C Memo CLIA. CLIA.
With the 01/09/2015 publication of the revised IG, microbiology laboratories will have 2 options for CLIA quality control: Follow all applicable CLIA quality control regulations. Implement IQCP. CLIA surveyors will cite laboratories for non-compliance. CLIA.
Amendment to the CLIA statute signed by the President on December 4, Clarifies that proficiency testing samples to be tested in the same manner as patient specimens, EXCEPT that no proficiency testing samples shall be sent to another laboratory for analysis. CLIA.
Imposition of the 2-year owner/operator prohibition when the laboratory is sanctioned for proficiency testing referral. CLIA.
Final rule details hierarchical adverse actions for proficiency testing referrals by seriousness. Defines when discretion will be applied and when revocation will be imposed. CLIA.
A second instance in which a proficiency testing sample, or a portion of a sample, is referred, for any reason, to another laboratory for analysis prior to the laboratory’s proficiency testing program event cut-off date within the period of time encompassing the two prior survey cycles. Applies to all CLIA certificate types. CLIA.
Repeat PT referral or laboratory sends PT samples to another laboratory and reports that laboratory’s test results as it’s own. CMS must impose: Revocation of the laboratory’s CLIA certificate. Civil Money Penalty (CMP) CMS may impose: Owner prohibition. Includes a provision for discretion in exempting the owner from the prohibition. CLIA.
Laboratory sends PT samples or the results of PT samples to another laboratory prior to, or on, the event cut-off date. Referring laboratory reports its own PT sample results. CMS must impose: Suspension/Limitation of the laboratory’s CLIA certificate. Civil Money Penalty (CMP) Directed Plan of Correction. CMS may impose: Any other alternative sanctions, as appropriate. CLIA.
Laboratory sends PT samples to another laboratory but no test results were receveid prior to the event cut-off date. CMS must impose: Civil Money Penalty (CMP) Directed Plan of Correction. CMS may impose: Any other alternative sanctions, as appropriate. CLIA.
One-time, narrow exception carve-out for intentional PT referral. Clarifies intentional PT referral carve-out with addition of the following terms/definitions: Reflex testing. Confirmation testing. Distributive testing. CLIA.
CMS will consider the referral to be improper, but not intentional, and subject to alternative sanctions in accordance with 42 CFR § (c): If the referral is not a repeat proficiency testing referral. It the referral is limited to reflex, confirmatory, or distributive testing. If the sample was a patient specimen, the referral would have been in full conformance with written, legally accurate and adequate standard operating procedures for the laboratory’s testing of patient specimens. CLIA.
Reflex Testing. Confirmatory or additional laboratory testing that is automatically requested by a laboratory under its standard operating procedures for patient specimens when the laboratory’s findings indicate test result that are abnormal, are outside a predetermined range, or meet other pre-established criteria for additional testing. CLIA.
Confirmatory Testing. Testing performed by a second analytical procedure that could be used to substantiate or bring into question the results of an initial laboratory test. CLIA.
Distributive Testing. Laboratory testing performed on the same specimen, or an aliquot of it, that requires sharing it between two or more laboratories to obtain all data required to complete an interpretation or calculation necessary to provide a final reportable result for the originally ordered test. When such testing occurs at multiple locations with different CLIA certificates, it is considered distributive testing. CLIA.
IQCP Mailbox: CLIA Interpretive Guidelines Mailbox: CLIA.
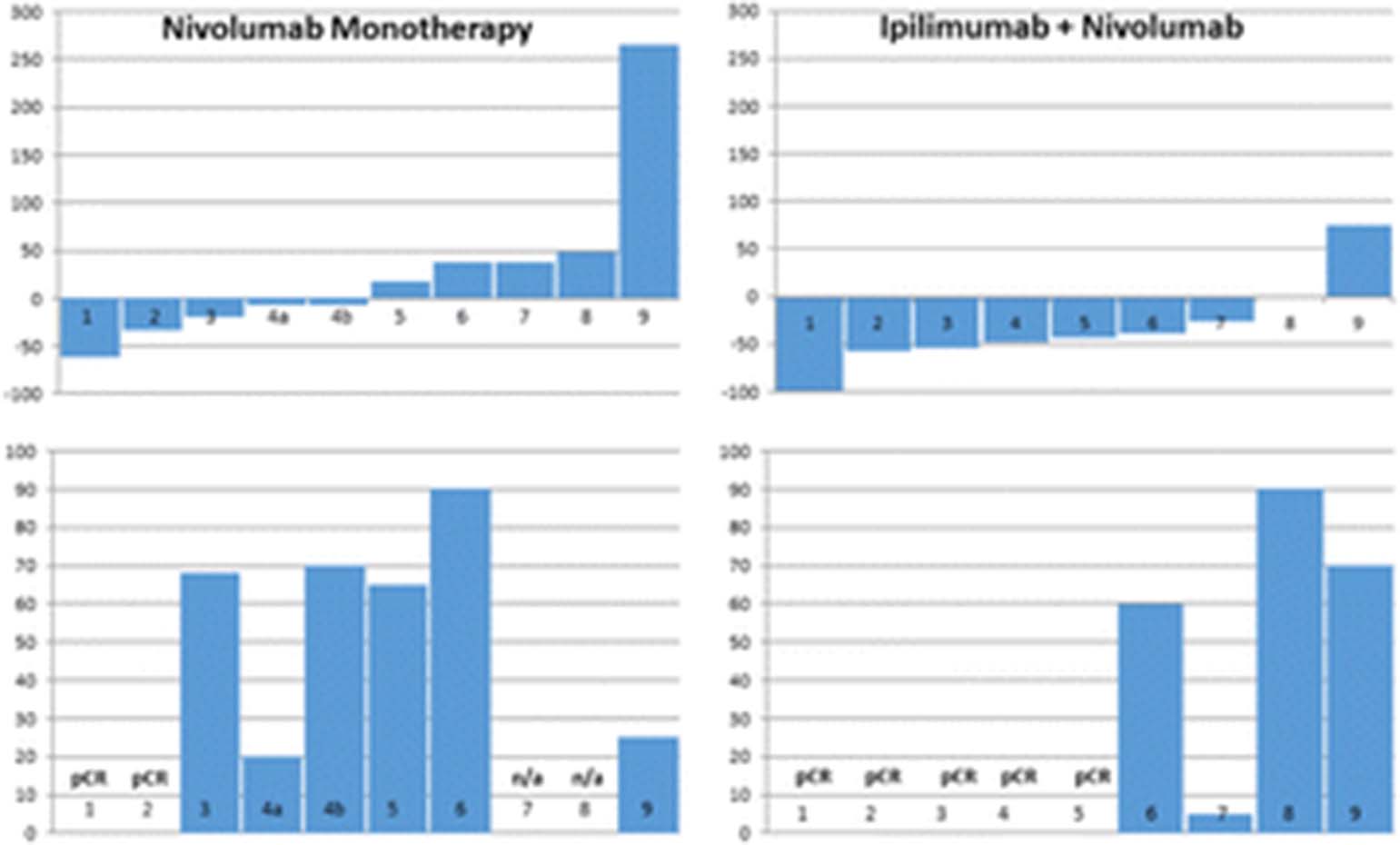
32nd Annual Meeting and Pre-Conference Programs of the Society for Immunotherapy of Cancer (SITC 2017): Part One

PPT - clia immunoassay PowerPoint Presentation, free download - ID:7715804
PPT - CLIA PowerPoint Presentation, free download - ID:573645

PDF) ACPA's 77th Annual Meeting Abstracts
PDF) Anatomy of the KISS Principle for Trainee Cleft Surgeons: The Quest for a Cleft Aesthetic Code of the Philtrum and Median Tubercle in Unilateral Cleft Lip Surgery using Strictly Anatomical Concepts

CLIA Hot Topics CLIA Gary Yamamoto - ppt download

pittcon 2013 technical program

USCAP 104th Annual Meeting by USCAP365 - Issuu

CLIA Hot Topics CLIA Gary Yamamoto - ppt video online download

Clia final regulations

CLIA Hot Topics CLIA Gary Yamamoto - ppt download

PPT - CLIA PowerPoint Presentation, free download - ID:573645

PPT - CLIA PowerPoint Presentation, free download - ID:573645
i.ytimg.com/vi/YGiXT5yqPIA/hq720.jpg?sqp=-oaymwEhC
Magnetic properties of rare-earth metallofullerenes
Introduction - Casida - 2001 - Pest Management Science - Wiley Online Library
PHI Team – Robert Byer NTT Profile and Scientific Papers List





